 |
|
|
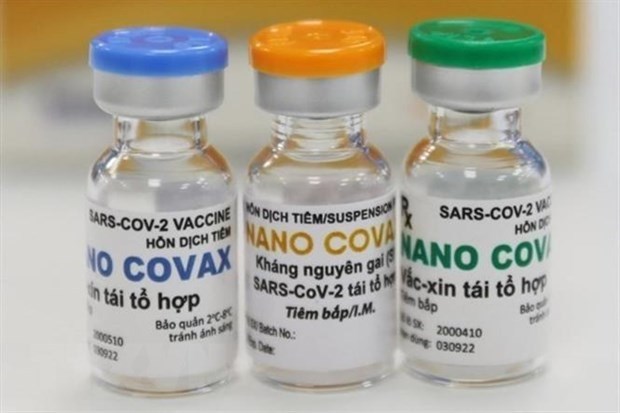
In Notice No. 248/TB-VPCP, which sums up the conclusion of the Deputy PM at a meeting on research, technology transfer, clinical trial and production of drugs, vaccines, and biological products, the Ministry of Health is required to give more support to COVID-19 vaccine research units in completing and supplementing dossiers of vaccines, first of all the Nano Covax, to submit to the National Committee for Ethics in Biomedical Research, and the Advisory Council for the Registration of Circulation of Drugs and Medicinal Ingredients for consideration and emergency approval .
Deputy PM Dam also asks the MOH to collect recommendations of the two councils’ members on the use of COVID-19 vaccines for children, the combination of vaccines, and plans for vaccine use in 2020.
The MoH is also asked to guide and help related firms in the trial of the anti-viral drug Molnupiravir, and design plans to ensure the availability of treatment drugs to meet the demand in the situation of rapid increases in COVID-19 patients, while speeding up the clinical trial and evaluation of the efficacy of the Vipdevir drug for COVID-19 treatment researched and produced by the Vietnam Academy of Science and Technology.
The ministry will also direct domestic units to continue researching medicine for COVID-19 treatment, coordinate will the Ministry of Science and Technology and relevant agencies in helping enterprises to soon produce rapid antigen testing kits which can be effective on many strains of SARS-CoV-2 virus./.
Source: VNA